Abstract
Background: Adult T-cell leukemia-lymphoma (ATLL) is a generally fatal malignancy caused by the human T-cell leukemia virus (HTLV-1), which is endemic in Japan, the Caribbean, and South America. ATLL is frequently encountered in South Florida afflicting immigrants (or their descendants) from neighboring Afro-Caribbean islands, South Americans (from Peru and Brazil mostly), and occasionally African-Americans. ATLL is generally incurable by conventional therapies. Clinical trials for ATLL have only yielded modest results. The median survival times for the most aggressive acute and lymphomatous forms in the modern era are <9 months and <11 months, respectively. The combination of zidovudine (ZDV) plus interferon (IFNα) can be efficacious in patients with leukemic ATLL forms, but response rates are still suboptimal as patients usually relapse and succumb to their disease, and molecular responses are not seen. Belinostat is a pan-histone deacetylase (HDAC) inhibitor that demonstrated dose-dependent apoptotic effects (augmented by ZDV) in our ATLL preclinical models, induced expression of the highly immunogenic HTLV-1 oncoprotein Tax, and blocked HTLV-1 basic zipper factor (HBZ), a key protein driving longevity of HTLV-1 infected T-cell clones. Based on these observations, we hypothesized that belinostat would reactivate HTLV-1 provirus in ATLL cells in subjects treated with ZDV (+/-IFNα), thus eliciting an anti-tumor immune response to help to eradicate minimal residual disease (MRD).
Methods: This phase 2 trial evaluates belinostat with ZDV and optional IFNα [standard or pegylated(peg)] as consolidation therapy in patients with aggressive ATLL who have persistent blood circulating ATLL at least 2 weeks after prior chemotherapy or ZDV-IFNα (Clinicaltrials.gov NCT02737046). The regimen consists of up to eight 21-day cycles of intravenous belinostat 1,000 mg/m2(Days 1-5), oral ZDV 300mg three times daily, and optional IFNα (5-10 million units daily, or pegylated form 180 mcg weekly) in patients who previously benefitted followed by maintenance ZDV (+/-IFNα) to complete at least 12 months of therapy. The primary objectives are to evaluate safety and complete molecular response rates by measuring T-cell receptor clonality using multiplex PCR. Secondary outcomes include clinical response rates, measuring molecular effects of belinostat in ATLL cells in vivo (histone acetylation, induction of Tax and disruption of viral latency, and effects on HBZ levels), HTLV-1 proviral loads, and cytotoxic T-cell responses.
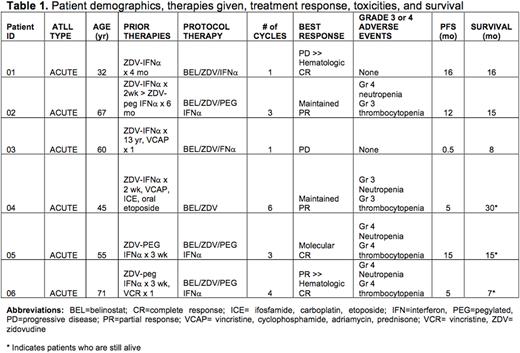
Results: Patient demographics, therapies given, toxicities, treatment response, and outcome measures are summarized in Table 1. Patients completed a minimum of 1 cycle, or a maximum of 6 cycles. Treatment was discontinued after recurring grade 3 or 4 hematologic toxicities (expected events) despite dose reductions as specified by the protocol (n=4 patients), or after progressive disease (PD) (n=2 patients). So far, clinical activity has been observed in 5 of 6 patients with acute type ATLL. Two patients (one heavily pre-treated, and one previously sensitive to ZDV-IFNα) had early PD with leukemia phase during cycle 1; however, one of these subjects, who appeared to have rapid leukemic progression after belinostat, underwent spontaneous hematologic remission after therapy was stopped with no MRD in blood compartment until relapse with only extra-nodal disease present at 16 months. In the latter subject, belinostat transiently increased histone acetylation in leukemic ATLL cells in vivo. Two additional patients achieved complete hematologic responses in peripheral blood and bone marrow compartments, of which one remains in molecular remission on dose-reduced weekly peg-IFNα at 15 months. Two patients maintained partial responses after 5 and 12 months, respectively.
Conclusions: These breakthrough clinical data demonstrate complete molecular response can be achieved in patients with acute type ATLL treated with belinostat and ZDV-IFNα (or peg-IFNα) alone, supporting our main hypothesis that judicious use of HDAC inhibitors may provoke sustained immune responses to help eradicate MRD. After fulfillment of the primary study endpoint (molecular remission in at least one subject), the study is expected to complete accruals (10 patients) in 2022. Complete results, including safety, efficacy, and correlative molecular studies will be presented at the meeting.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
Zidovudine for HTLV-1 related ATLL Pegylated interferon-alfa for HTLV-1 related ATLL
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal